Co-authored by Ms. Shristi Bansal, LexOrbis Associate and Mr. Mayank Jain, Fifth Year student of IP University, Delhi.
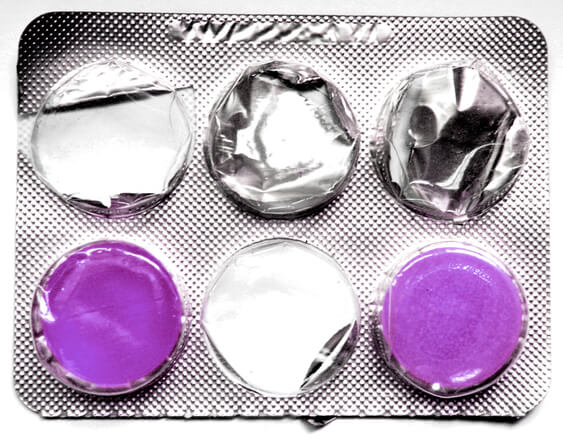
Pharma giants are often pitted against one another for various intellectual property controversies. This time, there has been a trademark dispute over color. Indian Pharma giant Dr. Reddy’s Laboratories has been temporarily prohibited from selling a generic version of the Nexium, a purple coloured gastronomical pill manufactured by the UK based AstraZeneca PLC.
In a lawsuit[1]filed before the Delaware Court, AstraZeneca alleged trademark infringement of their pill Nexium, used for heartburns and acid reflux. It was contended that Dr. Reddy’s generic version of the same is likely to create confusion amongst consumer due to the identical purple colour of the pills. AstraZeneca asserted that the colour purple has been extensively used to promote its pills via means like advertisements, radio and other consumer publications. As a result of such widespread promotions, the general public associates purple pills to AstraZeneca. Further, the pill was protected by three federal trademarks, including trademark over the phrase “The Purple Pill”
The Court, while discussing the concept of‘likelihood of confusion’ laid down the following as important factors:
- the degree of similarity between the owner’s mark and the alleged infringing mark;
- the strength of the owner’s mark;
- the price of the goods and other factors indicative of the care and attention expected by the consumers when making a purchase;
- the length of time the defendant has used the mark without evidence of actual confusion arising;
- the intent of the defendant in adopting the mark;
- the evidence of actual confusion;
- whether the goods, [even if] not competing, are marketed through the same channels of trade and advertised through the same media;
- the extent to which the targets of the parties’ sales efforts are the same;
- the relationship of the goods in the minds of the consumers because of the similarity function;
- other facts suggesting that the consuming public might expect the prior owner to manufacture a product in defendant’s market, or that he is unlikely to expand into that market.[2]
The Court, after giving weightage to the totality of circumstances, including the appearance of the pills granted an injunction against Dr.Reddy.
However, this was not the end. Dr. Reddy struck back by filing a counter suit in New Jersey Court claiming that filing this lawsuit was a material breach of a settlement which was entered into between the two pharmaceutical companies back in 2011[3]. As per this settlement, Dr. Reddy had been released from any liability with regard to the pill Nexium.
Over the past few years, the world has seen an outbreak of IP litigations involving giant pharmaceutical companies. Suits such as this one may be considered as pushing the limits of one’s IP right.While taking proactive actions against infringement of IP right is always recommended, one must also remember that suits such as the present one may affect consumers who are denied access to, even if temporarily, to generic version of essential drugs at cheap prices.
[1]AstraZeneca AB, AstraZeneca LP, and AstraZeneca Pharmaceuticals LP vs. Dr. Reddy’s Laboratories, Inc. , Civ. No. 15-988-SLR, available at http://www.scribd.com/doc/290877170/AstraZeneca-v-Dr-Reddys#scribd.
[2]Ibid
[3] “Dr Reddy’s sues AstraZeneca over purple colour of Nexium generic”, available at http://articles.economictimes.indiatimes.com/2015-11-20/news/68440284_1_dr-reddy-s-nexium-law-firm